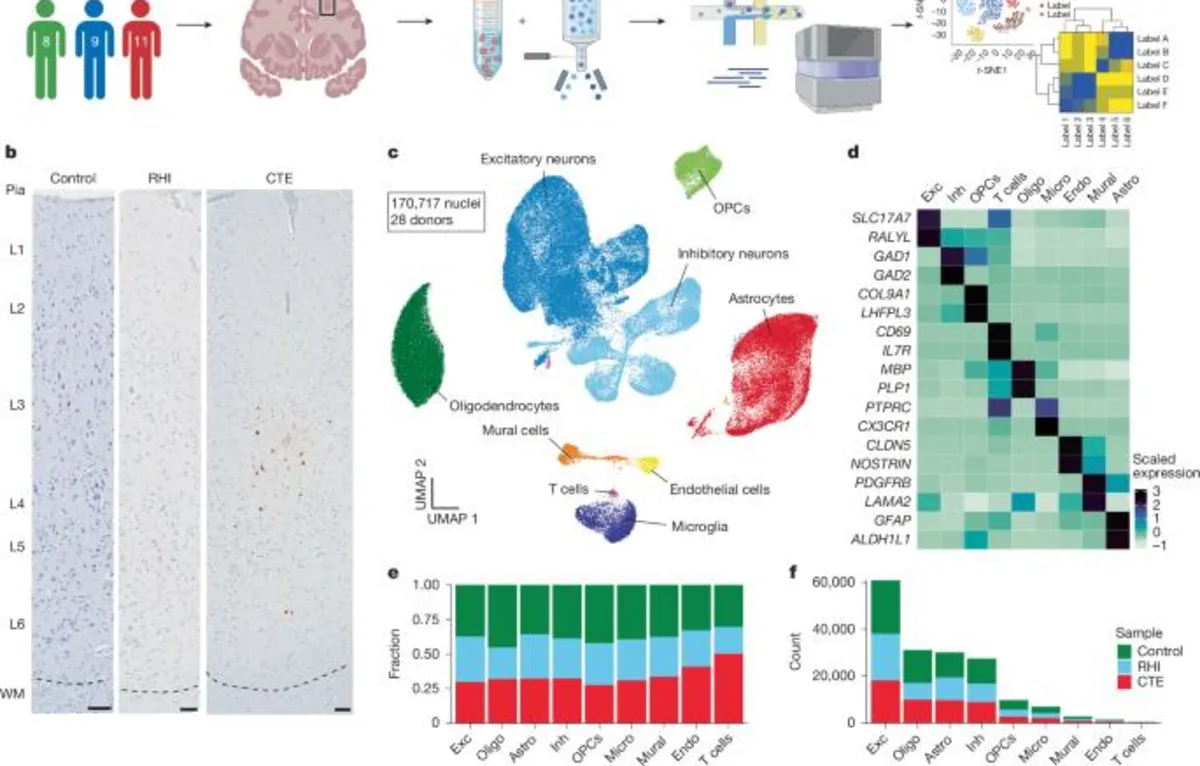
In our recent study, we investigated the role of microglial inflammation in chronic traumatic encephalopathy (CTE) and its significant contribution to neurodegeneration. By analyzing the gene expression profiles of microglial cells, we identified changes that may further our understanding of CTE pathology.
Our analysis encompassed a total of 6,863 microglial cells, leading to the identification of 11 distinct clusters (refer to Fig. 2a). The size of these microglial clusters aligns with findings from other published studies, confirming the robustness of our dataset. Among these, Cluster 10 consisted of 263 cells expressing perivascular macrophage genes such as CD163, F13A1, and LYVE1. In contrast, Cluster 6 contained 108 cells that expressed peripheral monocyte genes including PTPRC, LYZ, and CR1, consistent with previous observations.
Clusters 0, 2, 3, and 9 exhibited expression of classical homeostatic microglial genes, namely CX3CR1, P2RY12, and NAV2, and were categorized as homeostatic microglia. Notably, these homeostatic clusters showed a significant enrichment for nuclei from control individuals, in comparison to those with only repetitive head impact (RHI) or CTE, with statistical significance noted (control vs. RHI-only: P = 0.048; control vs. CTE: P = 0.047; RHI-only vs. CTE: P > 0.99).
The proportion of homeostatic microglia was observed to decline with an increase in years of football play (P = 0.004, β = −12.79). Conversely, Cluster 7 demonstrated heightened expression of CD83, CCL3, and HSP90AA1, suggesting a potential pro-resolving phenotype akin to findings in Alzheimer’s disease. Cluster 4, characterized by the highest differential gene expression of AIF1 (encoding IBA1), displayed notable expression of iron-associated genes like FTL and FTH1, along with ribosome-associated genes such as RPS24 and RPS11.
The microglial subpopulations present in RHI-only and CTE individuals significantly differed from those in control subjects, with the emergence of clusters 1, 5, and 8, collectively labeled as RHI microglia (RHIM) 1 to 3. Through gene module analysis with hdWGCNA and Celda, we identified co-expression patterns indicative of possible cellular pathways across these subclusters, employing linear mixed modeling for comparative analysis.
Our findings revealed that the expression of homeostasis-associated gene modules significantly decreased in RHIM2 and RHIM3, with RHIM1 (Cluster 5) expressing neuronal-associated genes like GRID2, GRIK2, and GRIA4, linking to synapse organization as identified in Gene Ontology (GO) terms.
Clusters RHIM2 and RHIM3 were almost evenly enriched across RHI and CTE samples, indicating a transcriptional profile suggestive of an inflammatory microglial phenotype. This was characterized by the expression of genes such as SPP1, HIF1A, TLR2, IL1B, and CTSB, with SPP1 serving as a marker for activated microglia and highlighting its potential role in synaptic engulfment in neurodegenerative contexts.
The analysis of RHIM2 and RHIM3 clusters revealed an increase in inflammation, hypoxia, and metabolic responses compared to homeostatic clusters, corroborating the findings from GO and DEG analyses. Distinct differences emerged between RHIM2 and RHIM3, with RHIM2 showing an increase in complement response markers, while RHIM3 was characterized by upregulation of hypoxia mediators HIF1A and VEGFA.
To validate the reduction in homeostatic microglial populations, we utilized IBA1 and P2RY12 for co-immunolabeling across 35 individuals with varying years of football play. Results indicated a significant decrease in homeostatic microglial densities with increased football exposure (P
We further validated the presence of RHIM2 and RHIM3 cells via in situ hybridization, labeling microglia expressing SPP1 and HIF1A. Across 21 individuals, the number of SPP1+HIF1A+ microglia in the cortical sulcus increased significantly with years of football play (P = 0.028), suggesting a regional specificity for this inflammatory phenotype.
We also compared our microglial populations against existing datasets, including a large dataset from Sun et al., which included over 100,000 microglia from more than 400 individuals. Our analysis confirmed significant correlation of RHIM2 and RHIM3 with inflammatory and stress-associated gene sets, further validating the consistency of our findings.
Our study suggests that exposure to repetitive head impacts induces an increase in inflammatory microglial states prior to the onset of CTE. The localized microglial inflammation observed in the sulcus of RHI-exposed individuals may play a crucial role in the initiation and maintenance of neuronal dysfunction and inflammation associated with CTE.
In summary, this comprehensive analysis highlights the significant alterations in microglial populations in response to RHI and their implications for understanding the underlying mechanisms of CTE.