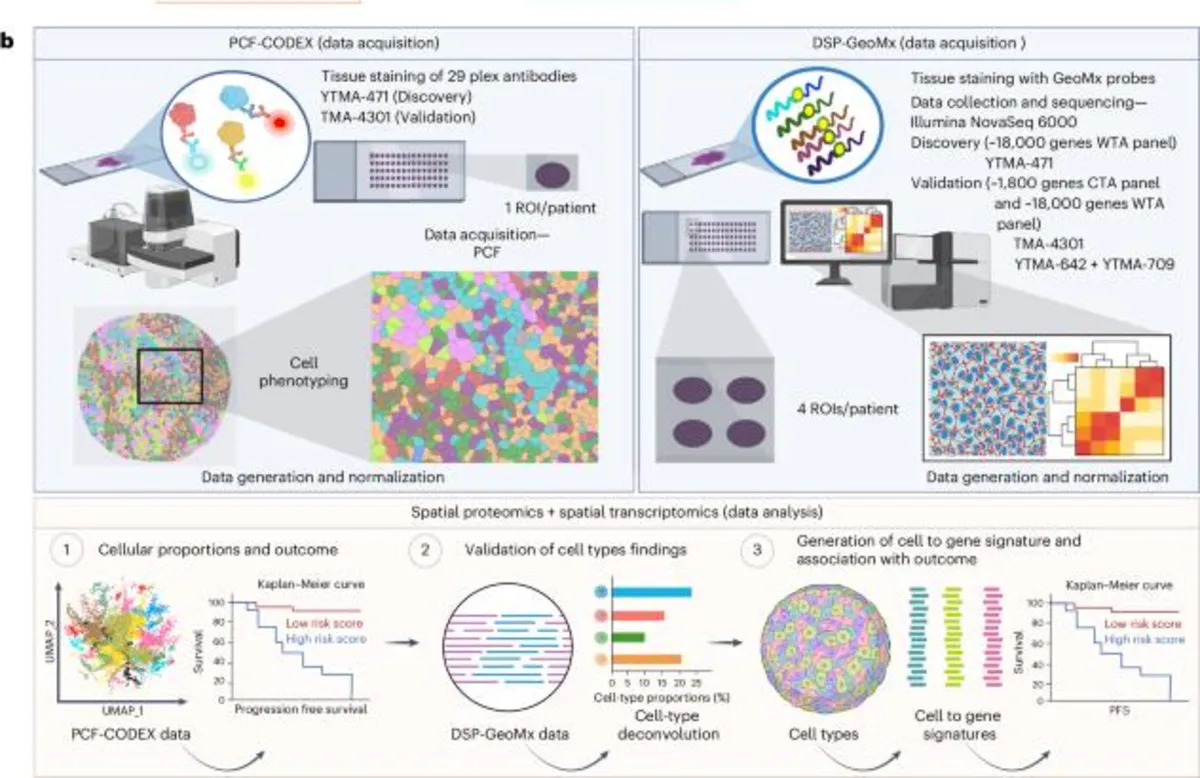
In the realm of advanced non-small cell lung cancer (NSCLC), spatial proteomic profiling has emerged as a powerful tool for understanding tumor and stromal cellular populations. This profiling has elucidated distinct cellular populations within tumor and stromal compartments, revealing significant insights into patient outcomes.
Figures 2a and Extended Data Fig. 1a,b showcase a comprehensive cell phenotyping approach utilizing a 29-marker panel. Specifically, Fig. 2b details the markers employed for identifying specific cell types, while representative tumor areas of interest (AOIs) are depicted in Fig. 2c and 2d. Notably, tumor cells were predominantly present in tumor AOIs, while M1/M2 macrophages and fibroblasts were found to be more abundant in stromal AOIs.
In our study, we compared cell-type distributions concerning PFS. Observations from tumor AOIs in both training (Fig. 2e) and validation cohorts (Fig. 2f) revealed that granulocytes and proliferating tumor cells were enriched in patients with shorter PFS. Conversely, in stromal AOIs (Fig. 2g,h), M1 and M2 macrophages were more prevalent among patients with longer PFS. These findings were consistently replicated across cohorts.
We further analyzed cell composition in the Yale cohort, applying a 5-year PFS cut point. However, due to limited follow-up data, a similar analysis was not feasible for the UQ cohort. The observed patterns aligned with previous studies indicating that granulocytes and proliferating tumor cells are enriched in progressors within the tumor compartment, while M1 and M2 macrophages dominate in nonprogressors within the stromal compartment (Supplementary Fig. 1a,b).
To delve deeper into the associations between cell fractions and treatment outcomes, we employed a 2-year PFS endpoint, conducting univariable Cox analyses for each individual cell type and compartment. In the tumor compartment, granulocytes, proliferating tumor cells, and vessel cells were linked to an increased risk of disease progression or death (Fig. 3a and Supplementary Fig. 2a). Although these trends occurred across cohorts, they did not achieve statistical significance following Benjamini–Hochberg (BH) adjustment.
Conversely, the stromal compartment exhibited associations where M1 macrophages, M2 macrophages, and CD4 T cells correlated with improved PFS in both cohorts (Fig. 3c and Supplementary Fig. 2b). While consistent trends were noted, including the negative association of granulocytes, vessels, and proliferating tumor cells with PFS, and the positive association of M1 and M2 macrophages, none remained significant post-BH adjustment.
We also investigated associations with 5-year PFS in the Yale cohort (Supplementary Fig. 2c,d), which revealed consistent patterns with the 2-year analysis across both tumor and stromal compartments.
Utilizing spatial proteomic-derived cell fractions, we developed spatial cell-type signatures to predict outcomes to immunotherapy in advanced NSCLC patients. A comprehensive overview of the signature generation pipeline is illustrated in Extended Data Fig. 2. The Yale cohort served as the training set, which was systematically split into multiple tenfolds. We constructed least absolute shrinkage and selection operator (LASSO)-penalized Cox models to predict 2- and 5-year PFS, while also identifying resistance-associated cell types by enforcing their coefficients to be non-negative.
Cross-validation across splits yielded a final Cox regression model trained on cell types consistently selected, pinpointing proliferating tumor cells, vessels, and granulocytes as high-risk features. Notably, the resistance model in the tumor compartment demonstrated a significant association with worse PFS (HR = 3.8, P = 0.004, two-sided log-rank test; Fig. 3d,e). Further validation in the UQ cohort corroborated this predictive value (HR = 1.8, P = 0.05, one-sided log-rank test; Fig. 3f).
In the stromal compartment, while granulocytes and proliferating tumor cells were predictive in the training set (Supplementary Fig. 3a,b), they did not reach significance in the UQ validation cohort (Supplementary Fig. 3c), despite similar trends being observed.
To explore the spatial features linked to resistance and response models derived from cell frequencies, we analyzed the spatial organization and interactions of immune cells. Voronoi diagrams illustrated the spatial distribution of cell types associated with both response (Fig. 4a) and resistance (Fig. 4b) models. M2 macrophages were predominantly located in response-associated regions, whereas the resistance diagram exhibited an enrichment of vessels, PD-L1+ tumor cells, and proliferating tumor cells.
Cellular neighborhoods were constructed to understand the spatial aggregation of cellular communities. Our analysis identified ten distinct neighborhoods, annotated based on predominant cell-type enrichment. Neighborhood frequencies were correlated with PFS, revealing that proliferating tumor communities were more common in patients who experienced disease progression (P = 0.07, two-tailed t test), further supporting the role of tumor proliferation in therapy resistance (Fig. 4d).
The analysis of pairwise spatial interactions uncovered significant cell-cell relationships. Proliferating tumor cells exhibited predominant interactions with granulocytes and endothelial cells, suggesting their roles in tumor proliferation and angiogenesis. In the response model, interactions among M1/M2 macrophages or CD4 T cells with other immune or tumor cells demonstrated robust immune regulation, highlighting a potential avenue for therapeutic intervention.
The response model emphasized the critical role of M1 and M2 macrophages and CD4 T cells in predicting responses to PD-1-based immunotherapy. UMAP visualizations depicted the expression dynamics of all 14 cell types, showing higher PD-L1 expression on M1 (CD68+) and M2 (CD163+) macrophages compared to PanCK+Ki67+ proliferating tumor cells. Notably, nonprogressors within the Yale cohort demonstrated significantly elevated PD-L1 expression in both tumor cells and macrophages.
However, comparative analysis revealed that PD-L1 expression on macrophages was consistently associated with longer PFS, while expression on tumor cells did not yield the same significance. This finding was further supported by regression analysis, indicating a strong correlation between PD-L1 expression on macrophages and PFS, underscoring the potential predictive value of macrophage PD-L1 expression in treatment outcomes.
To validate our proteomic findings, we conducted cell-type deconvolution using whole transcriptomic data. Utilizing CIBERSORTx with the LM22 gene signature matrix, we observed increased M2 macrophage fractions in patients with longer PFS. Statistical analysis confirmed a substantial increase in M2 macrophages correlating with better outcomes, reinforcing the role of these immune cells in predicting favorable responses to immunotherapy.
Collectively, these findings highlight the importance of spatial cell-type signatures in understanding the tumor microenvironment and their potential for guiding treatment strategies in advanced NSCLC patients.