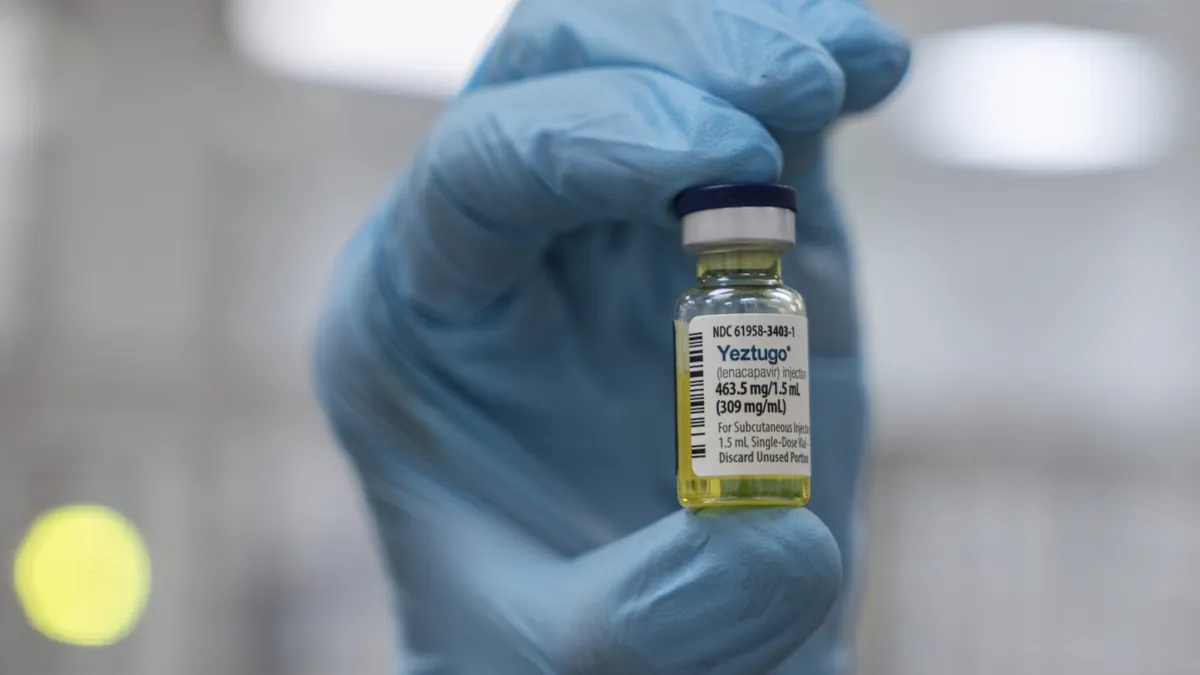
In a significant development in the fight against HIV, the U.S. has officially approved the world’s only twice-a-year shot to prevent the virus, as announced by Gilead Sciences on Wednesday. This innovative treatment is expected to play a crucial role in a global rollout that could offer protection to millions of individuals at risk of HIV infection. However, the extent of access to this powerful new option remains uncertain both in the U.S. and internationally.
While the search for a viable HIV vaccine continues, many experts believe that the newly approved shot, known as lenacapvir, may serve as a highly effective alternative. Research indicates that lenacapvir has demonstrated remarkable results, nearly eliminating new infections among high-risk populations in two landmark studies. This method could surpass the effectiveness of daily preventive pills, which individuals may forget to take. Greg Millett, public policy director at amfAR, The Foundation for AIDS Research, emphasized, “This really has the possibility of ending HIV transmission.”
Condoms remain a reliable defense against HIV infection when used correctly. However, PrEP (pre-exposure prophylaxis) — the regular use of preventive medications, such as daily pills or a different shot administered every two months — is gaining significance in combating the virus. With lenacapvir offering six months of protection, it stands out as the longest-lasting option available, appealing to individuals who may be hesitant about frequent doctor visits or the stigma associated with daily pills.
Despite the promising potential of lenacapvir, various challenges could hinder its distribution. A turbulent landscape in U.S. healthcare, characterized by budget cuts to public health agencies and reductions in Medicaid, raises concerns about the accessibility of this new treatment. Millett expressed that the “gaping holes in the system” could complicate efforts to ensure that individuals not only receive lenacapvir but also return for follow-up doses, which are required even just twice a year.
Gilead’s lenacapvir is already marketed as a treatment for HIV under the brand name Sunlenca. The preventive dosage will be available under a different name, Yeztugo. The administration involves two injections under the skin of the abdomen, creating a small “depot” that allows the medication to gradually absorb into the bloodstream. Although the exact price for the new preventive shot has not been announced, it’s important to note that lenacapvir specifically prevents HIV transmission and does not protect against other sexually transmitted diseases.
Global initiatives aimed at ending the HIV pandemic by 2030 have encountered obstacles, with over 30,000 new infections occurring annually in the U.S. and approximately 1.3 million globally. Currently, only about 400,000 Americans utilize some form of PrEP, which is a mere fraction of the estimated number who would benefit from it. Recent studies have shown that regions with high PrEP usage have witnessed a decrease in HIV infections, whereas rates continue to rise in other areas.
Notably, around half of new HIV infections occur in women, who often require methods of protection that do not necessitate their partner’s knowledge or consent. A rigorous study conducted in South Africa and Uganda involving over 5,300 sexually active young women and adolescent girls found that those receiving the lenacapvir shot reported no HIV infections, in stark contrast to about 2% of those using daily pills who contracted the virus from infected partners.
A second study indicated that the twice-yearly lenacapvir shot was nearly as effective among gay men and gender-nonconforming individuals in the U.S. and several other countries heavily impacted by HIV. Ian Haddock, a participant in the lenacapvir study from Houston, noted that he had previously tried PrEP intermittently but eagerly joined the study and continues with the twice-yearly shots. “Now I forget that I’m on PrEP because I don’t have to carry around a pill bottle,” he stated. Haddock, who leads the Normal Anomaly Initiative supporting Black LGBTQ+ communities, emphasized the broad applicability of this prevention method for all individuals, regardless of gender or sexual orientation.
Dr. Gordon Crofoot of Houston, who played a pivotal role in the study for men, stressed the need for universal access to effective PrEP. “Everyone in every country who’s at risk of HIV needs access to PrEP,” he asserted. He called for improved accessibility to highly effective preventive options, such as lenacapvir, to enhance global health outcomes.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.