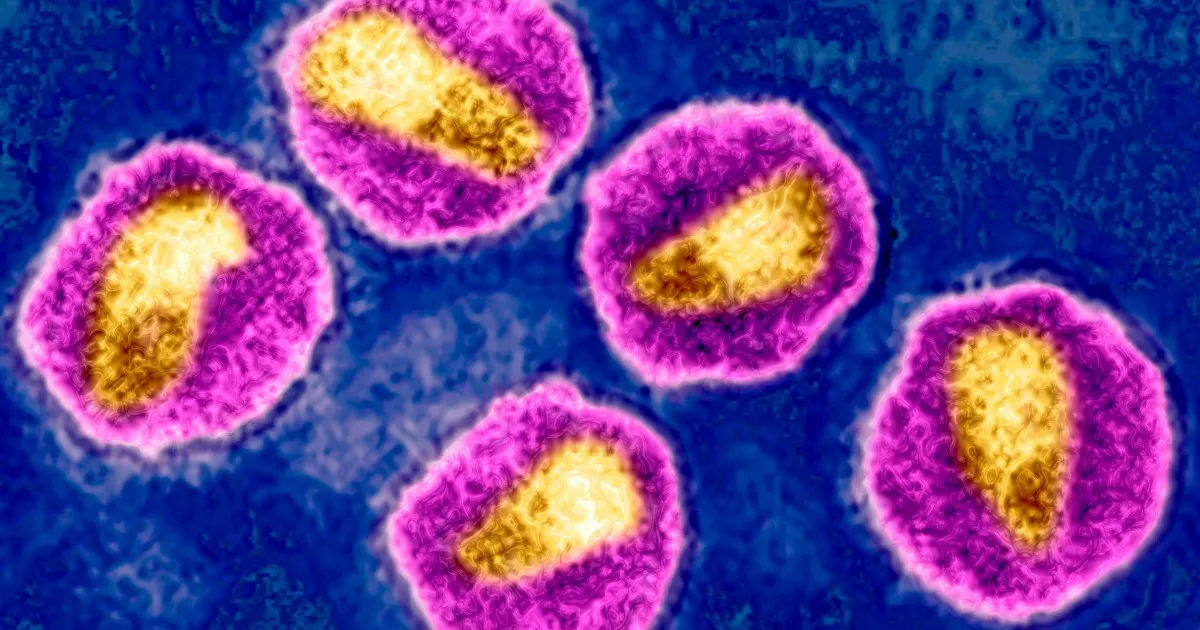
LONDON — The European Medicines Agency (EMA) has made a significant recommendation for public health by endorsing the authorization of a revolutionary injectable drug, lenacapavir, aimed at preventing HIV transmission. This twice-yearly injectable treatment, marketed as Yeytuo by Gilead Sciences, could potentially play a crucial role in ending the spread of the virus. In an official statement released on Friday, the EMA highlighted that thorough evaluations of lenacapavir demonstrated it to be “highly effective” and deemed it “of major public health interest.”
Once the European Commission accepts the EMA's guidance, lenacapavir will be authorized for use across all 27 EU member countries, as well as in Iceland, Norway, and Liechtenstein. This broad authorization aims to enhance the accessibility of the drug to those at risk of HIV in Europe.
Recent studies conducted last year revealed that lenacapavir, which is already utilized in the treatment of individuals living with HIV, was nearly 100% effective in preventing transmission among both women and men. Winnie Byanyima, the executive director of the U.N. AIDS agency, emphasized that this breakthrough drug could significantly alter the course of the HIV epidemic if made accessible to those who need it most.
Earlier this year, in June, the U.S. Food and Drug Administration (FDA) authorized lenacapavir as a preventative measure against HIV. Additionally, the World Health Organization (WHO) recently recommended that countries offer this drug as an alternative option for individuals at risk of contracting the virus.
While traditional methods such as condoms can effectively guard against HIV infection when used correctly, lenacapavir presents a unique advantage with its six-month protection duration, making it the longest-lasting preventive treatment available. This lengthy interval could appeal to individuals who are hesitant about frequent visits to healthcare facilities or who may face stigma associated with taking daily pills.
Despite the promising prospects of lenacapavir, critics have voiced concerns regarding the potential limitations in its global availability, which might hinder efforts to combat widespread HIV outbreaks. Gilead Sciences has announced plans to permit the sale of affordable, generic versions of lenacapavir in 120 low-income countries with high HIV prevalence, predominantly located in Africa, Southeast Asia, and the Caribbean. However, this initiative notably excludes nearly all of Latin America, where HIV rates, although lower, are on the rise. This exclusion raises alarms that the world may be missing a vital opportunity to curtail the HIV epidemic.
According to UNAIDS, approximately 630,000 deaths due to AIDS were recorded globally last year, with over 40 million individuals estimated to be living with HIV. The introduction of lenacapavir could be a pivotal step towards changing these statistics and improving public health outcomes across the globe.