At the functional core of life, a complex interplay exists between nucleic acids and proteins, yet the origins of this relationship remain elusive. Nucleic acids, such as DNA and RNA, play critical roles in storing, replicating, and transmitting genetic information through their inherent structural properties that facilitate molecular self-recognition. In contrast, proteins serve as the molecular, structural, and catalytic workhorses necessary for sustaining life.
Unlike nucleic acids, peptides lack the innate ability to replicate in a sequence-specific manner, necessitating a method to control and transmit the peptide sequences essential for survival. This control is achieved through the encoding of peptide sequences in nucleic acids. Understanding how nucleotide-controlled peptide biosynthesis initially emerged represents a significant gap in our comprehension of life's origins, presenting formidable challenges due to the complexity of protein synthesis.
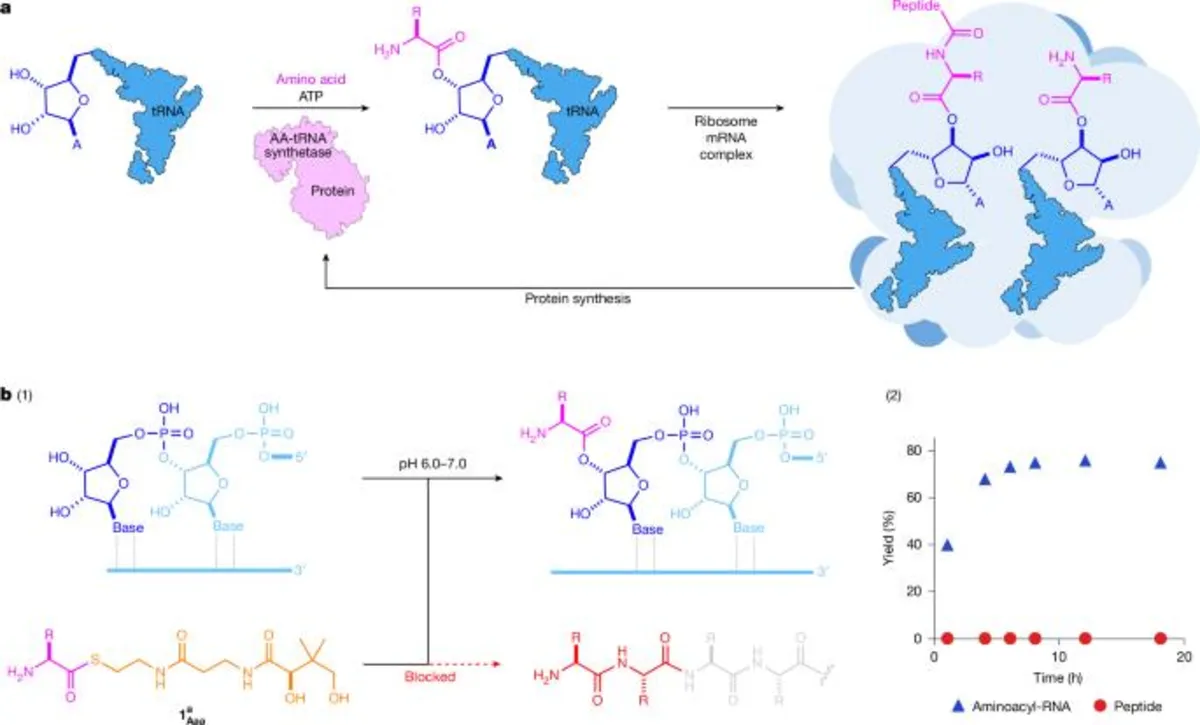
All proteins are synthesized through a universally conserved two-stage process known as ribosomal peptide synthesis (RPS). In the first stage, transfer RNAs (tRNAs) are aminoacylated, effectively loading activated amino acids onto the 2′,3′-diol moiety of the tRNA. This step is subsequently followed by ribosome-catalyzed peptide synthesis. A fundamental aspect of RPS involves the orchestration and control of activated amino acids through their covalent attachment to RNA, suggesting that the selective formation of RNA esters is foundational to RPS.
In biological systems, RNA aminoacylation is regulated by a unique family of synthetase enzymes. Interestingly, these enzymes themselves are synthesized by the ribosome using the very same genetic code they enforce, creating a paradox regarding the origins of RNA aminoacylation. Despite its significance, discovering a non-enzymatic pathway for synthesizing aminoacyl-RNA has long been a challenge, as RNA amino acid esters are not known to form selectively in water.
To address the challenge of selective RNA aminoacylation, researchers have examined various electrophiles, such as aminoacyl phosphates, aminoacyl imidazole, and N-carboxyanhydrides (NCAs). However, these compounds are highly unstable in aqueous environments and do not react selectively with the RNA 2′,3′-diol, leading to uncontrolled background amide formation. Therefore, achieving controlled RPS requires the selective formation of aminoacyl-RNAs, a feat that remains elusive.
In the quest for a solution, researchers hypothesized that biological thioesters could serve as an ideal activation mode for achieving selective RNA aminoacylation in water. Thioesters are integral to central metabolism, driving numerous anabolic pathways, including those for fatty acids, polyketides, and non-ribosomal peptide synthesis. Both thioesters and RNA aminoacylation possess deep-rooted biochemical origins, predating the last universal common ancestor of all life on Earth.
This research demonstrated that biological aminoacyl-thiols could chemoselectively aminoacylate RNA at neutral pH in aqueous solutions. This non-enzymatic RNA aminoacylation highlights a significant chemical distinction between RNA aminoacylation and peptide synthesis stages, showcasing how these two steps can be chemically controlled under identical conditions in water.
Throughout these experiments, it was crucial to understand the stability and reactivity of the ambiphilic aminoacyl-thiol. Interestingly, it was found to be stable in water without undergoing polymerization. The reaction of aminoacyl-thiols with various nucleophiles revealed selective aminoacylation at the 2′,3′-diol of ribonucleosides, even in the presence of large excesses of competing nucleophiles.
These findings suggest that RNA's ability to form duplexes may play a critical role in directing the site of aminoacylation, which is essential for proper protein synthesis. The research further indicates that the selective aminoacylation of RNA at the 3′-terminus is vital for ribosomal peptide synthesis, enabling the translation of genetic information into functional proteins.
Understanding the origins of aminoacyl-thiols and their role in RNA aminoacylation could pave the way for insights into the early evolution of peptide synthesis. The ability to achieve selective aminoacylation in water using thioesters opens new avenues for exploring the biochemical pathways that may have led to the emergence of life on Earth.
In summary, the intricate relationship between nucleic acids and proteins is central to the foundation of life. The ongoing research into nucleic acid aminoacylation through thioester activation not only enhances our understanding of protein synthesis but also sheds light on the evolutionary processes that underpin the origins of life itself.