In the popular video game The Last of Us and its acclaimed HBO spin-off series, humanity fights for survival against the ominous threat of cordyceps, a parasitic fungus that transforms its hosts into zombie-like creatures. While these portrayals are dramatized for entertainment, recent scientific findings reveal that these fungi are not merely a product of fiction. A groundbreaking study suggests that some species of cordyceps have existed since the age of the dinosaurs.
An international team of researchers, led by Yuhui Zhuang, a doctoral student of paleontology at Yunnan University in China, has made a remarkable discovery. They found two cordyceps-infected insects preserved in 99-million-year-old amber. The fossils, which include a fly and an ant pupa, represent some of the oldest known records of animal-pathogenic fungi, dating back to the Cretaceous period. This significant finding was published in the journal Proceedings of the Royal Society B on June 11.
“Overall, these two fossils are extremely rare, especially among the tens of thousands of amber specimens we’ve examined. Only a select few have preserved the symbiotic relationship between fungi and insects,” Zhuang remarked in an interview with CNN.
The amber, sourced from northern Myanmar, has been the center of violent conflict since 2017, largely due to a surge in fossil amber research. However, the study clarifies that the specimens used were acquired prior to this period and were not connected to any conflict.
To analyze the fossilized insects, Zhuang and his colleagues employed optical microscopes and created 3D images using a technique known as micro-computed tomography. This advanced imaging revealed unexpected aspects of the insects’ infections.
The researchers discovered that both of the newly identified fungal species belong to the genus Ophiocordyceps, which includes the notorious zombie-ant fungus. This particular fungus is known for its ability to manipulate its host's behavior. During the final stages of infection, the fungus takes control of the insect's brain, compelling it to seek elevated areas that receive ample sunlight and warmth—ideal conditions for spore production.
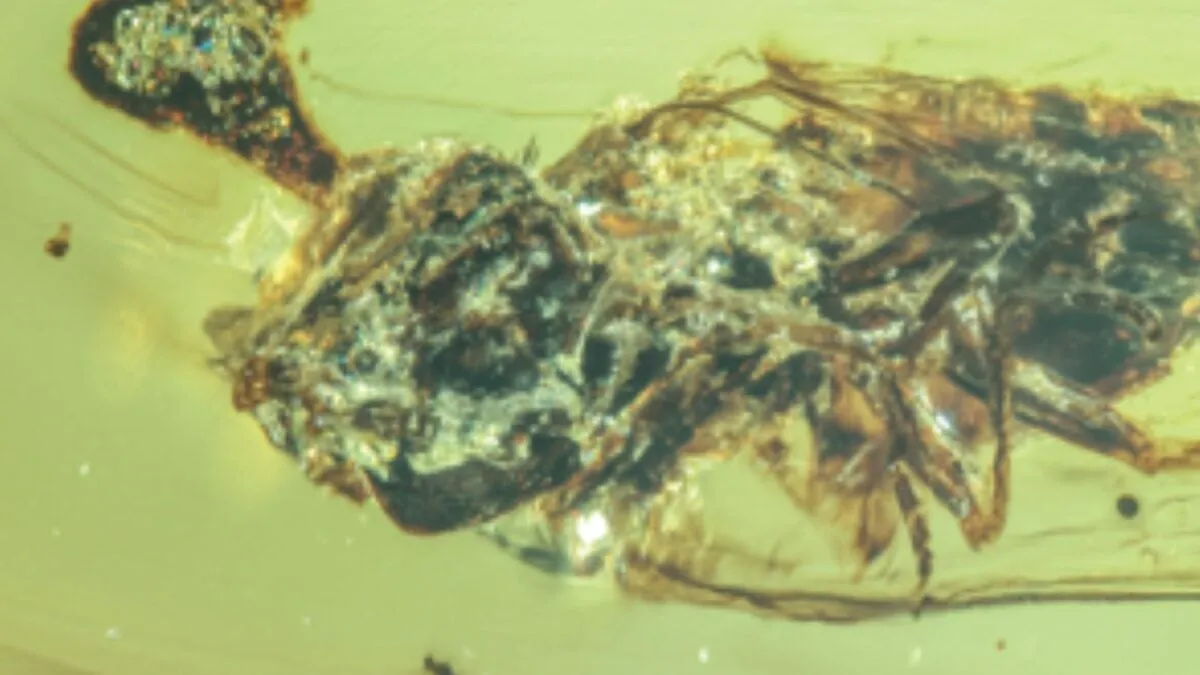
Once the host insect dies, a fungal growth emerges from its head, releasing spores that can infect new victims. The fossilized fly, in particular, was found with the fruiting body of P. ironomyiae protruding from its head. Interestingly, unlike typical Ophiocordyceps infections that yield a smooth, swollen fruiting body, this species exhibited an unexpanded, textured growth.
The ant pupa, infected with P. gerontoformicae, displayed even more atypical characteristics. Instead of the fungus emerging from the pupa’s head, it erupted from the metapleural gland, which is responsible for producing antimicrobial secretions. This phenomenon has not been observed in any known species of Ophiocordyceps, indicating that these fungi are indeed novel species.
Through comparative analysis of these fungi's structures and growth patterns with known Ophiocordyceps species, researchers identified distinct traits that linked them to the genus, yet found no matches with existing species. They utilized DNA from contemporary Ophiocordyceps species to construct a phylogenetic tree, illustrating the evolutionary history of the genus. This analysis suggested that Ophiocordyceps originated in the early Cretaceous period, initially infecting beetles before evolving to target butterflies, moths, and eventually other insects including bees and ants.
The study concluded that the diversification and abundance of insect host species during the Cretaceous likely fueled the rapid emergence of new Ophiocordyceps species, shedding light on the complex interactions between fungi and their hosts throughout history.