A groundbreaking research team from the University of Basel, Switzerland, has unveiled an innovative molecule designed to mimic plant photosynthesis. This remarkable molecule can store two positive and two negative charges simultaneously when exposed to light, aiming to enhance the conversion of sunlight into carbon-neutral fuels.
Plants harness the energy of sunlight to transform carbon dioxide (CO2) into energy-rich sugar molecules through a process known as photosynthesis. This natural mechanism is fundamental to life on Earth, as it allows animals and humans to metabolize carbohydrates, releasing energy and producing carbon dioxide, thereby completing the cycle. The new research seeks to emulate this process, potentially leading to the creation of environmentally friendly fuels.
The research team is focused on developing high-energy compounds, known as solar fuels, such as hydrogen, methanol, and synthetic gasoline. If successfully produced and utilized, these fuels would emit only the amount of carbon dioxide necessary for their creation, making them carbon-neutral. This could represent a significant advancement in sustainable energy solutions.
In a recent publication in the journal Nature Chemistry, Professor Oliver Wenger and his doctoral student Mathis Brändlin discussed a critical milestone in the quest for artificial photosynthesis. They have engineered a unique molecule capable of storing four charges—two positive and two negative—simultaneously under the influence of light. This capability for intermediate charge storage is essential for converting sunlight into chemical energy, enabling reactions such as the splitting of water into hydrogen and oxygen.
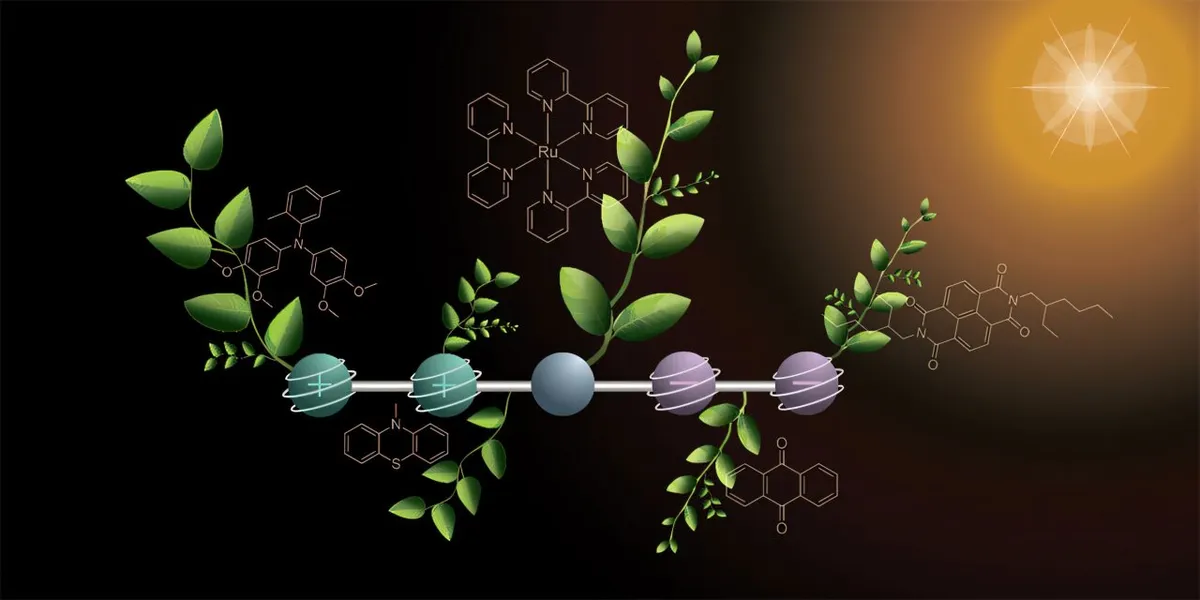
The newly developed molecule consists of five interconnected parts, each responsible for a specific function. One side features two components that release electrons, becoming positively charged in the process, while the opposite side contains two parts that capture electrons, thus turning negatively charged. At the center of the molecule, a special component is designed to absorb sunlight and initiate the crucial reaction known as electron transfer.
To generate the four charges in the molecule, the researchers adopted a two-step process using two separate flashes of light. The initial flash triggers a reaction that produces one positive and one negative charge, which then move to opposite ends of the molecule. The second flash repeats this reaction, resulting in the molecule ultimately housing two positive and two negative charges.
This innovative stepwise excitation method allows the use of significantly dimmer light, bringing the process closer to the intensity of natural sunlight. "We are already moving close to the intensity of sunlight," explains Brändlin. Previous studies relied on extremely powerful laser light, which strayed far from the practical vision of artificial photosynthesis. Additionally, the charges within the molecule maintain stability long enough for subsequent chemical reactions to occur.
While the new molecule has yet to be integrated into a fully functioning artificial photosynthesis system, it represents a pivotal advancement. "We have identified and implemented an important piece of the puzzle," states Wenger. The insights gained from this study enhance our comprehension of the electron transfer processes central to artificial photosynthesis. The researchers remain hopeful that their findings will pave the way for innovative solutions in creating a sustainable energy future.