In the pursuit of advancing neural repair, we have adopted a five-step pipeline designed to streamline the identification of candidates for effective therapies, potentially leading to human testing (see Fig. 1a). Each step of this process plays a crucial role in ensuring that the selected compounds exhibit beneficial biological effects, particularly in the context of spinal cord injuries (SCI).
The first step of our pipeline focuses on characterizing the transcriptomic state or ‘signature’ of a specific cell type known for its regenerative capabilities. In our study, we examined the transcriptome of corticospinal motor neurons, which have shown the ability to regenerate following SCI. Previous research indicated that within two weeks post-injury, these neurons undergo a temporary transcriptional reversion to an embryonic state, allowing them to regain their regenerative potential. This valuable data is accessible to the public for further research.
In Step 2, we conducted an in silico screen to evaluate the regenerating corticospinal transcriptome against shifts induced by over 1,300 small molecules cataloged in the Broad Connectivity Map (CMap). This resource provides insights into how various compounds affect the transcriptome of cultured cell lines. We searched for compounds that produced transcriptomic alterations similar to those observed in regenerating corticospinal neurons, thereby identifying potential candidates for further evaluation.
Moving to Step 3, we took our top candidates from the in silico screen and tested them using a medium-throughput in vitro assay on cultures of adult motor cortex neurons. This system, recently made available, represents a reliable predictive model for assessing potential in vivo effects in the mature nervous system, as it utilizes adult neurons rather than embryonic or early postnatal cells. Previous in vitro studies often relied on cell cultures that do not accurately reflect the responses of adult central nervous system (CNS) neurons, making our adult neuronal cultures pivotal for clinical translation.
Step 4 involves in vivo validation of the top drug candidate identified from our in vitro screen within an SCI model. Here, we utilized adult Fischer 344 rats subjected to severe contusive cervical SCI. After allowing a clinically relevant recovery period of two weeks, we categorized the animals into treatment groups to evaluate the effects of the candidate compounds on functional recovery.
Finally, Step 5 encompasses the validation of candidate compounds in cultures of adult primate cortical neurons, including human neurons. This step is crucial due to the limited availability of normal human neuronal resources. For this study, normal human neurons were obtained from surgical specimens during tumor resections. The emphasis on this step underscores the importance of assessing the clinical relevance of our findings.
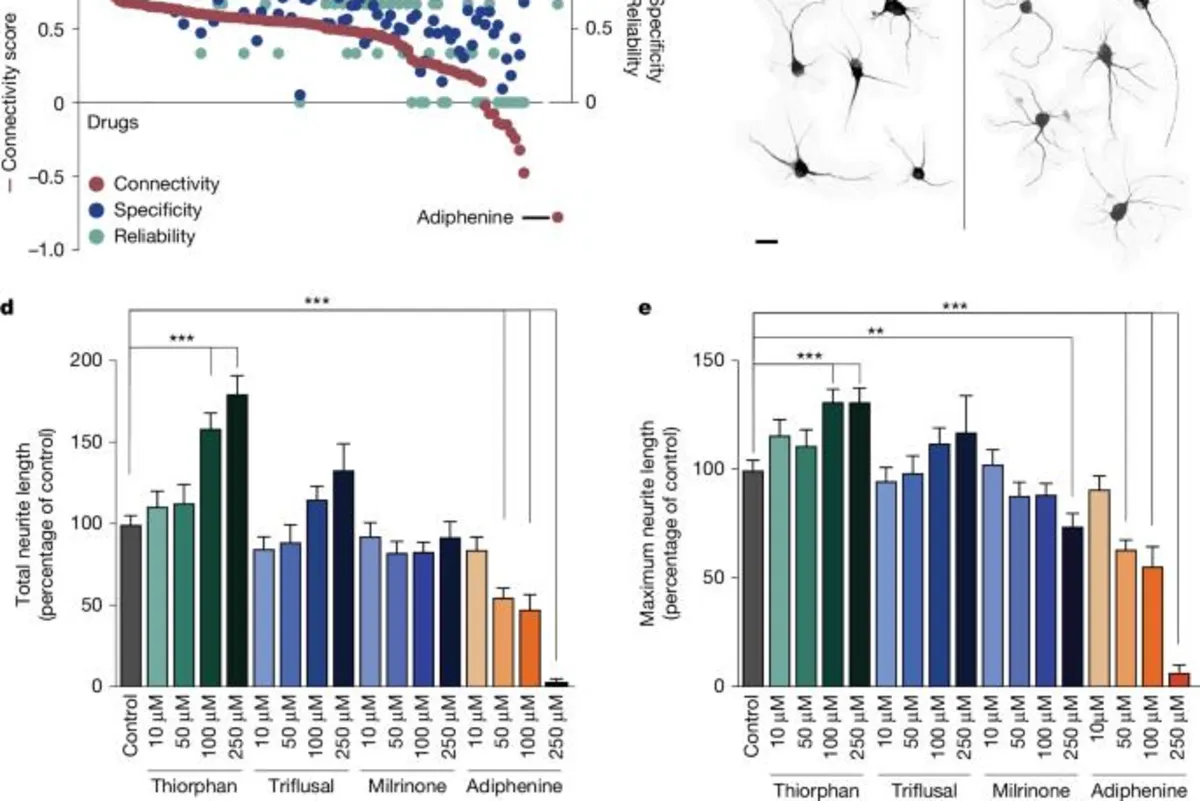
In our in silico analysis, we followed the methodology established by Lamb et al., identifying several compounds that closely matched the expression profile of the regenerating corticospinal system (see Fig. 1b and Supplementary Fig. 1a). We calculated a connectivity score ranging from +1 to -1 to describe the similarity between the corticospinal transcriptomic profile and that of individual compounds, with +1 indicating the highest similarity. Additionally, we established a reliability score for each compound, considering factors such as P value, false detection rate (FDR), and instance count.
The top compounds that emerged as ‘hits’ based on their enrichment, reliability, and specificity scores included: quinostatin, thiorphan, triflusal, and milrinone. Quinostatin, although not available for purchase, demonstrated the highest enrichment score. Thiorphan, primarily recognized as a neutral endopeptidase inhibitor, has been linked to neuroprotective effects, making it a strong candidate for further exploration.
We evaluated the lead candidate compounds through dissociated cultures of the adult mouse motor cortex, observing significant differences in neurite outgrowth and maximum neurite length post-exposure to various compounds. Notably, thiorphan emerged as the most effective compound, enhancing total neurite outgrowth by 80% at a dose of 250 μM compared to controls, demonstrating its potential in promoting neural repair.
Given the promising results from the in vitro screen, thiorphan progressed to Step 4, where we conducted in vivo testing in a model of SCI. Following a severe contusive injury, we administered thiorphan in conjunction with neural progenitor cell (NPC) grafts. The combination treatment showed a remarkable twofold improvement in forelimb grasping success compared to lesioned controls, underscoring the therapeutic potential of thiorphan.
To further understand the mechanisms underlying thiorphan's efficacy, we examined the regeneration of corticospinal axons into the grafts. Results indicated a significant increase in axon regeneration, confirming the beneficial effects of thiorphan infusion alongside NPC grafts. Importantly, thiorphan administration did not lead to any observable damage or neuronal loss in the motor cortex, affirming its safety profile.
In the final stage, we assessed thiorphan's effects on neurons isolated from the monkey M1 motor cortex and human cortical neurons. Treatment with thiorphan resulted in significant increases in both total neurite outgrowth and maximum neurite length, affirming its biological activity across species. These findings highlight the potential of thiorphan as a therapeutic candidate for enhancing neural repair in various contexts.
In conclusion, our five-step pipeline has successfully identified and validated thiorphan as a promising candidate for enhancing neural repair after spinal cord injuries. The integration of in silico analysis, in vitro assays, and in vivo testing underscores the comprehensive approach needed to advance therapeutic strategies in the field of regenerative medicine.