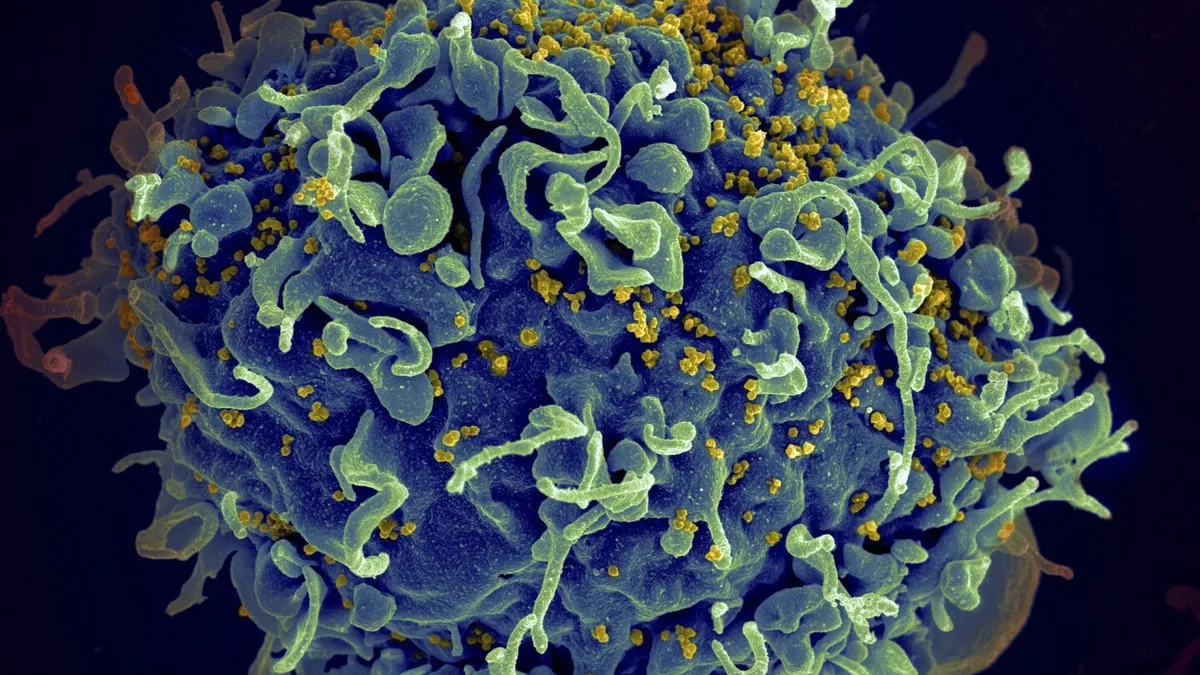
The European Medicines Agency (EMA) has taken a significant step towards combating HIV by recommending the authorization of a new injectable drug called lenacapavir, marketed as Yeytuo in Europe by Gilead Sciences. This twice-yearly injectable medication is designed to prevent the transmission of HIV, and scientists believe it could play a crucial role in ending the virus's spread.
In a statement released on Friday, the EU drug regulator highlighted that the evaluations of lenacapavir confirmed its high efficacy and classified it as a drug of “major public health interest.” Once the EMA's guidance is approved by the European Commission, lenacapavir will be authorized for use in all 27 EU member countries, along with Iceland, Norway, and Liechtenstein.
Research conducted last year indicated that lenacapavir, which is already utilized for treating individuals living with HIV, demonstrated nearly 100% effectiveness in preventing transmission among both women and men. Winnie Byanyima, the executive director of the U.N. AIDS agency, emphasized that this drug “could change the trajectory of the HIV epidemic” if it becomes accessible to everyone in need.
In June, the U.S. Food and Drug Administration (FDA) granted approval for lenacapavir as a preventive measure against HIV. Furthermore, earlier this month, the World Health Organization (WHO) recommended that countries provide lenacapavir as an additional option for individuals at risk of contracting the virus.
Lenacapavir’s unique six-month protection period positions it as the longest-lasting preventive option available. This extended duration could appeal to individuals who are hesitant about frequent health clinic visits or who face stigma associated with taking daily pills. Conventional preventive measures, such as condoms and daily pills, include another injectable drug, cabotegravir, which requires administration every two months.
Despite the promising nature of lenacapavir, critics have voiced concerns regarding its potential availability on a global scale. Drugmaker Gilead has announced plans to permit affordable generic versions of lenacapavir to be sold in 120 economically disadvantaged countries with high HIV prevalence, primarily located in Africa, Southeast Asia, and the Caribbean. However, Latin America is notably excluded from this initiative, raising alarms that the global community may be overlooking a critical opportunity to curb the HIV epidemic.
According to UNAIDS, approximately 630,000 AIDS-related deaths occurred worldwide last year, with an estimated 40 million individuals currently living with HIV. Byanyima has previously suggested that collaboration between U.S. leadership and Gilead could facilitate the production and licensing of lenacapavir, enabling access to millions in need across the globe.
The developments surrounding lenacapavir are being closely monitored, with the hope that this innovative prevention method will contribute significantly to reducing HIV transmission rates globally.
The Associated Press Health and Science Department is supported by the Howard Hughes Medical Institute’s Department of Science Education and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.