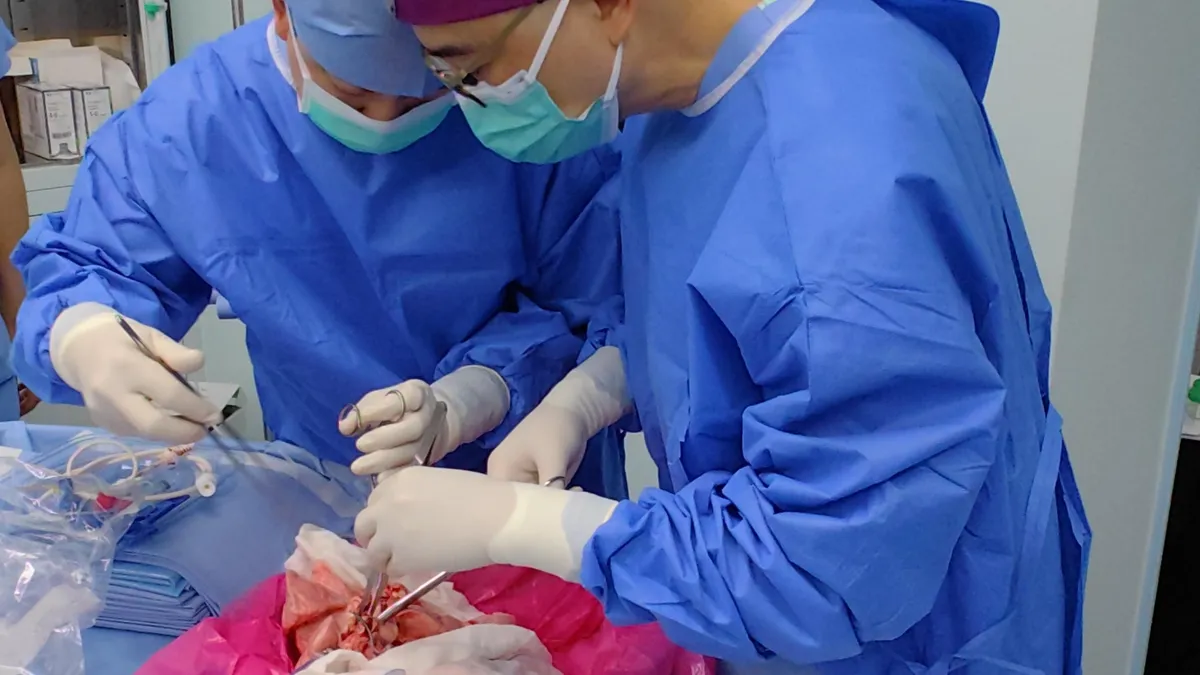
In a first-of-its-kind experiment, doctors in China successfully transplanted a lung from a genetically modified pig into a brain-dead individual. This pioneering procedure, conducted in Guangzhou, marks a significant advancement in the field of xenotransplantation. While similar experiments involving organs like kidneys and hearts have occurred in the U.S., this is the first instance of a lung being transplanted from another species into a human.
Prior to this groundbreaking lung transplant, various xenotransplantation experiments had been performed, including a notable case in China involving a pig liver. These innovations have laid the groundwork for potential transplants of pig organs into living human patients. However, so far, only a limited number of such procedures have been executed.
The experiment, described in the journal Nature Medicine on August 25, involved a 39-year-old male patient who was declared brain-dead before the procedure began. The medical team confirmed the patient's brain-dead status through four distinct assessments. They also obtained written informed consent from his family to proceed with the lung transplant.
Dr. Jiang Shi, a co-author of the study and a physician in the Department of Organ Transplantation at the First Affiliated Hospital of Guangzhou Medical University, emphasized the significance of this accomplishment, stating, "For our team, this accomplishment is a meaningful beginning."
Lung xenotransplantation presents unique biological and technical challenges compared to other organ transplants. The primary goal of this study was to investigate how the human immune system reacts to such a transplant, rather than to claim that the technique is ready for clinical use. This means that while the research is promising, it remains in the preclinical investigation stage.
Once transplanted, the pig lung demonstrated viability and functionality for a total of nine days, despite showing signs of rejection just 24 hours after the operation. The experiment concluded on Day 9 at the request of the patient’s family. Although it is uncertain how much longer the lung could have functioned, it did exhibit damage by the end of the nine-day period.
Dr. Adam Griesemer, a senior member of the xenotransplantation team at NYU Langone's Transplant Institute, remarked, "Nobody would sign up for a nine-day lung transplant." He underscored the importance of conducting studies with brain-dead individuals, as animal models may not accurately predict outcomes in human recipients.
The lung used in this study came from a genetically modified pig, with the editing performed by Clonorgan Biotechnology in Chengdu, China. Utilizing the CRISPR gene-editing technology, three pig genes were disabled to prevent the activation of the human immune system. Additionally, three human genes were introduced to enhance tolerance of the organ within a human body.
On May 2024, the transplant team surgically removed the left lung of the pig and implanted it into the brain-dead patient, who retained his right lung. To mitigate the risk of rejection, immunosuppressive drugs were administered starting one day before the procedure and continued daily after the operation.
The patient’s body did not exhibit signs of hyperacute rejection immediately after the transplant, which would have indicated an immediate immune response. However, by the third day, the immune system began generating antibodies against the transplanted organ, leading to swelling and inflammation.
The researchers proposed that future attempts at lung xenotransplantation may benefit from strategies aimed at blocking specific immune cells and suppressing inflammatory signaling molecules. The challenges associated with lung transplants are particularly complex, given their continuous exposure to external air, making them vulnerable to immune defense mechanisms.
Several questions remain regarding how to improve the approach and its long-term viability in living patients. Dr. Richard Pierson, a thoracic transplant surgeon at Massachusetts General Hospital, noted that it remains unclear how effectively the pig lung could support a patient removed from life-support systems.
In conclusion, this groundbreaking study provides critical insights into the immune, physiological, and genetic barriers that need to be addressed in the field of xenotransplantation. The authors believe that this research paves the way for further innovations and potential advancements towards clinical applications in the future.