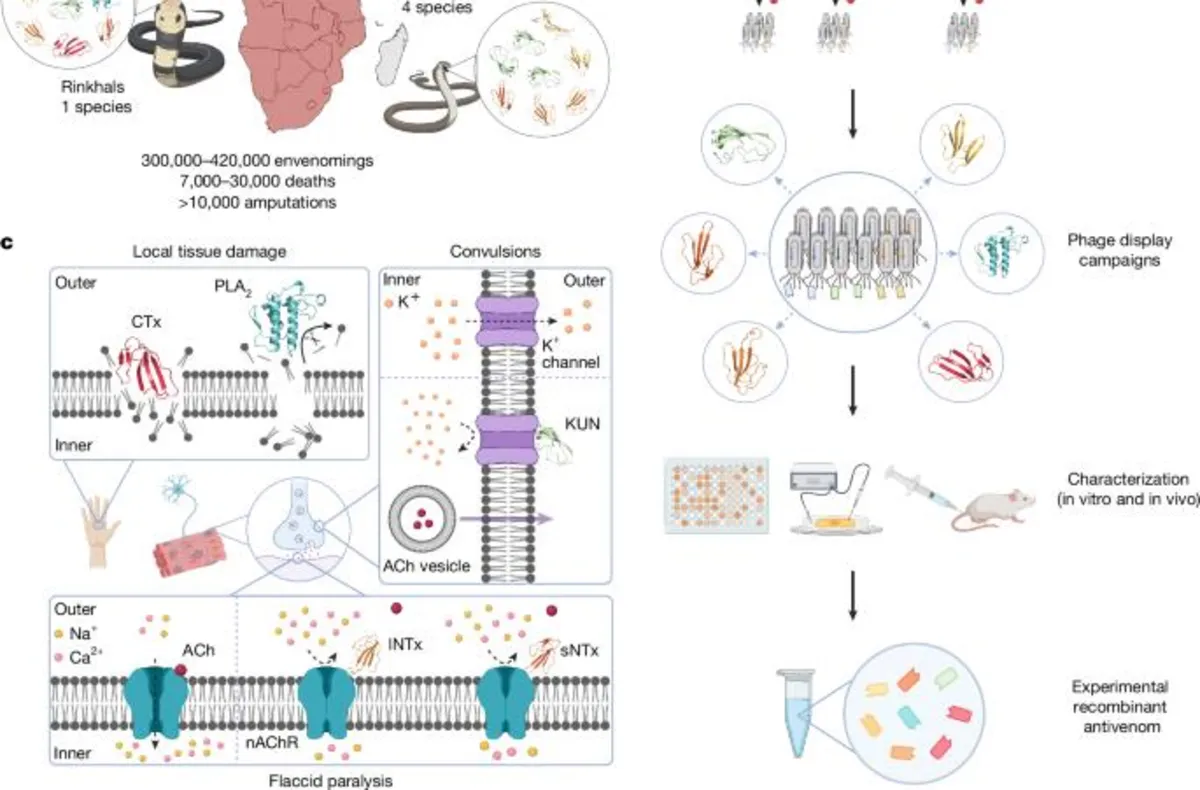
Recent research has revealed significant advancements in the development of polyvalent recombinant antivenoms utilizing VHHs (variable domain of heavy chain antibodies) to combat the complexities of snake venoms. Prior studies have successfully identified broadly neutralizing antibodies against snake toxins using methods such as phage display and yeast display technology. These techniques have demonstrated that rodents can be rescued from the effects of neurotoxin-dominated venoms through the use of these antibodies. However, these earlier findings primarily served as proof of principle rather than definitive solutions for clinical application.
While these studies showcased the potential of neutralizing antibodies, they primarily focused on single toxin families and relied heavily on individual monoclonal antibodies or VHHs to neutralize entire venoms. A significant limitation is that all but three studies concentrated on only one or two toxin families, which does not reflect the diverse challenges posed by snake venoms in real-world scenarios. Our current study addresses these limitations by demonstrating how a experimental polyvalent recombinant antivenom can be developed using just eight VHHs, targeting toxins from seven different protein subfamilies, thus achieving comprehensive coverage of continent-wide elapid species.
Our findings reveal the in vivo efficacy of this experimental antivenom on 18 different elapid venoms using the WHO-recommended pre-incubation model. We further confirmed its effectiveness on 11 of the venoms in a more rigorous rescue model that closely mimics actual snakebite scenarios. Notably, this recombinant antivenom outperformed the commercial plasma-derived antivenom, Inoserp PAN-AFRICA, in preventing dermonecrosis and lethality across all tested snake species, with the exception of D. polylepis.
By demonstrating that an efficacious polyvalent recombinant antivenom can be constructed using only eight VHHs, we challenge the prevailing belief that only polyclonal antibodies are effective in neutralizing the complexity of snake venoms. This work directly addresses a longstanding question in toxinology regarding the minimum number of antibodies necessary to develop a clinically useful recombinant antivenom.
Our research not only focuses on mortality rates but also emphasizes the effectiveness of the recombinant antivenom in mitigating dermonecrosis caused by venoms from spitting elapid snakes such as N. nigricollis, N. mossambica, and H. haemachatus. Although traditional plasma-derived antivenoms have shown efficacy in pre-incubation models, they are seldom tested in rescue models, which is critical for real-world applicability. Moreover, the rapid administration of antivenoms is essential for preventing severe local tissue damage, a challenge that our VHHs address effectively.
The choice to utilize the VHH format for our recombinant antivenom presents multiple advantages. Firstly, VHHs have a low risk of causing severe immunological reactions due to their typically low immunogenicity. This characteristic allows for earlier treatment initiation, circumventing the need for clinical syndromes to manifest. Secondly, VHHs display remarkable biophysical stability, promising a longer shelf-life, which is advantageous for stockpiling in various conditions. Thirdly, their small size allows for rapid distribution from the bloodstream to surrounding tissues, enhancing their neutralizing capacity at the bite site.
Despite the numerous advantages of VHHs, challenges such as their short half-life in circulation could limit their effectiveness over time. However, their ability to quickly penetrate deep tissue may allow for effective toxin neutralization before systemic circulation occurs. Future studies involving large animal experiments are necessary to explore the pharmacodynamics of VHHs and the toxicokinetics of venom components. If required, strategies such as repeated dosing or engineering techniques to extend VHH half-life could be employed.
While our study focuses on developing a defined oligoclonal mixture of VHHs against African elapid venoms, the strategies employed for antibody discovery and therapeutic prototype design hold promise for broader applications. This approach could be valuable in clinical contexts requiring the targeting of multiple isoforms across both closely and distantly related protein families, potentially extending its use to advanced therapies against infectious diseases and complex immunological disorders.
In conclusion, our research marks a significant step forward in the development of a recombinant antivenom capable of neutralizing a diverse array of snake venoms. By utilizing a small number of VHHs, we have not only demonstrated the potential for effective treatment options but have also opened avenues for further research and application in other medical fields.